 |

 |
| |
|
Contents:
African-American AIDS Conference Full, Available on Web Lipodystrophy: Glaxo Researchers Suggest Mechanisms, Publish Data New Antiviral Substances Found with HCG Health GAP Coalition--New Group for International Treatment Access Medical Marijuana: Major Report Released March 17 California: 9th Annual Lobby Day, April 19 San Francisco Area: Important Cyclosporin Study Recruiting
African-American AIDS Conference Full, Available on Web By John S. James
 Eighteen years into the epidemic, the first national medical
conference on African-Americans and AIDS, held in Washington
D.C. on February 25 and 26, admitted over 600 people, but had
to tell hundreds of others that it was full.
Eighteen years into the epidemic, the first national medical
conference on African-Americans and AIDS, held in Washington
D.C. on February 25 and 26, admitted over 600 people, but had
to tell hundreds of others that it was full.
A surge of interest shortly before the meeting filled all the space in the auditorium, and no room was available at nearby hotels for a video overflow session. At this time the conference Web site is getting 15,000 visitors per day. The Web coverage, located on the Johns Hopkins AIDS site, includes complete audio and slides of almost all the speakers. Particularly useful are the brief written summaries of the talks, each about a half page of text; these can be used to decide what sessions to listen to, or for an overview of the conference (even if one's computer cannot receive the audio).
Major areas of interest included medical care in prison, and how physicians can work on AIDS with African-American churches. The opening address was given by Donna Shalala, U.S. Secretary of Health and Human Services. An important talk which may not have a summary on the Web is "The Disconnect Between American Healthcare and the African- American Community," by Robert Fullilove, Ph.D. Using the book "I Heard It Through the Grapevine: Rumor in African- American Culture" by Patricia A. Turner (University of California Press, available in paperback, 1994), Dr. Fullilove illustrated the survival value and indeed necessity of folklore in a hostile society, and the importance of establishing credibility when disinformation has often been used for social control. Professionals fighting conspiracy theories are often frustrated that they cannot simply replace a non-fact with a fact in somebody's belief system, ignoring the larger context of the belief, and the fact that folklore can be one of the most important survival instincts for a community. [This analysis may help to understand the striking parallels between separate conspiracy theories which seem to have evolved independently in the African-American and in the gay communities--AIDS created in a government laboratory to get rid of African-Americans, or AIDS as an anti-gay myth, a monstrous fraud that is killing people by poisoning them with AZT and other drugs.] An expert round-table consensus meeting was held the day before, with a court reporter who prepared full transcripts in time for distribution on the second day of the conference. The transcript is not on the Web at the time of this writing, but may be placed there later. The speakers need a chance to edit their remarks first, to fix errors in the transcription. The "1999 National Conference on African-Americans and AIDS: A National Forum on HIV/AIDS for Health Professionals Who Provide Care for African-Americans" was sponsored by Johns Hopkins University, Harvard AIDS Institute, Howard University School of Medicine, the Institute of Human Virology at the University of Maryland, and the Rollins School of Public Health of Emory University; it was funded by Bristol-Myers Squibb. Later this year there will be report-backs from the conference in several major cities, but the schedule is not set yet. Next year's conference will take place February 24- 25, 2000, at the Renaissance Washington, D.C. Hotel, in a larger auditorium. The Johns Hopkins Web site is at http://www.hopkins-aids.edu. At this time the conference itself and the written summaries have separate links from the home page; it is easy to miss one or the other. Lipodystrophy: Glaxo Researchers Suggest Mechanisms, Publish Data By John S. James
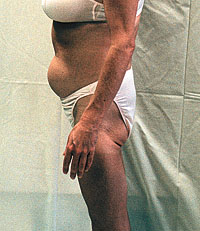 At the recent Retroviruses conference (Chicago, Jan. 31 -
Feb. 4), a research team from Glaxo Wellcome presented two
posters proposing possible mechanisms for the lipid (fat)
abnormalities which appear to be associated with use of
protease inhibitors in some patients--together with
supporting biochemical data from laboratory studies.(1,2)
At the recent Retroviruses conference (Chicago, Jan. 31 -
Feb. 4), a research team from Glaxo Wellcome presented two
posters proposing possible mechanisms for the lipid (fat)
abnormalities which appear to be associated with use of
protease inhibitors in some patients--together with
supporting biochemical data from laboratory studies.(1,2)
Other mechanisms have been proposed, but without such laboratory data. Unfortunately the posters had limited impact at the conference, because this research is based on lipid biochemistry which is unfamiliar to most AIDS experts. We have met with the researchers and received additional documentation to help us explain this work to a larger audience. Part of the difficulty in research on lipodystrophy is that pharmaceutical companies have disincentives to highlight problems with their products. Glaxo has different incentives, because it has not marketed a protease inhibitor yet--and the lipid research suggest that its protease inhibitor amprenavir (Agenerase(TM), which was originally developed by Vertex Pharmaceuticals Inc. and formerly known as VX478 or 141W94), may not have the same problem. Also, according to Glaxo, there has been almost no lipodystrophy in its expanded-access program which so far has given the drug to over 1500 patients. But no one knows if these laboratory results will predict what happens in people; and the expanded-access program has not given amprenavir to enough people for long enough to know with confidence that they will not have as much lipodystrophy as those treated with other drugs, since this problem is most likely to occur after long-term use. While these results are interesting, only time will tell whether this drug will be associated with less lipodystrophy than other HIV protease inhibitors in human use. Mechanisms No one knows for sure what is causing symptoms such as "Crix belly" or "buffalo hump" and associated loss of fat in the face, arms, and legs--and metabolic complications which are often associated, including high triglycerides and cholesterol, and development of insulin resistance or even diabetes in some patients. Most experts today believe that protease inhibitors are probably involved, even though there were a few such cases before these drugs were used. Many researchers believe that the fundamental problem in this syndrome is the loss of subcutaneous fat, rather than its abnormal accumulation. The loss of equilibrium between fat deposited in the body and lipids in the bloodstream may lead to very high blood levels of LDL cholesterol (the "bad cholesterol") and triglycerides. For unknown reasons, this excessive fat in the bloodstream can be deposited in certain parts of the body, causing abnormal fatty growth there--and also increasing the long-term risk of cardiovascular disease. In addition, the loss of fat due to certain illnesses is associated with a predisposition to insulin resistance--the relative ineffectiveness of insulin in the body, which can lead to diabetes if it becomes severe enough. The Glaxo Wellcome researchers who did these studies had much previous experience in measuring lipid changes, through studies of other conditions including diabetes and obesity. They applied tests they were already familiar with to look for effects of HIV protease inhibitors on fat cells in the laboratory. Their work suggests that not all lipodystrophy is the same, but that there are at least two distinct mechanisms involved, depending on which protease inhibitor is used. (In both cases the tests were done with animal cells--a reminder that this work is still a theory of lipodystrophy, not a proven cause.) One Possible Mechanism: Preventing Development of Fat Cells In one experiment,(1) fat cells were grown in the laboratory, with or without HIV protease inhibitors. In this test, saquinavir, ritonavir, and nelfinavir greatly reduced the development of fat cells from stem cells; however, neither indinavir nor amprenavir had much effect. Saquinavir, ritonavir, and nelfinavir also increased the metabolic destruction of fat in existing fat cells. Another Mechanism: Increasing Retinoid Toxicity Retinoids are compounds related to vitamin A; some of them are found naturally in the body, where they have many different effects. Too much of certain retinoids causes toxic effects due to abnormal biochemical signaling in the body, and the toxicity of excessive vitamin A can resemble some of those sometimes seen in the lipodystrophy syndrome. The same animal cells used above were also tested in this experiment.(2) But here, instead of measuring the fat cells produced in a laboratory culture, the researchers used a certain gene in these cells, which is sensitive to retinoid signaling and also produces a product which is easy to measure in the laboratory. The protease inhibitors alone did not affect the activity of the gene. When protease inhibitors were combined with retinoids the results were complex, depending on the protease inhibitor and the retinoid. But perhaps the most important single result is that indinavir (alone among all the protease inhibitors in human use) clearly stimulated signaling by all-trans retinoic acid (ATRA). ATRA is a retinoid produced naturally in the body from vitamin A. While it is too early to know that these laboratory studies apply to humans, the researchers suggest that indinavir may cause some lipodystrophy problems by changing retinoid signaling--in effect, causing retinoid toxicity not by increasing the amount of ATRA present, but by increasing the body's sensitivity to it. If this theory is correct (and no one knows yet), it would suggest that vitamin A supplementation might be harmful if one is taking indinavir, by making some of the lipodystrophy problems more likely to occur. Possibly this theory could be tested by looking for correlations between lipodystrophy and vitamin A intake in clinical databases--if there are databases which have enough patients taking indinavir, keep consistent records on lipodystrophy, and record nutritional intake. This theory is related to the one published by Carr and others last summer,(3) but there are biochemical differences. References 1. Lenhard J, Weiel J, Paulik M, Miller L, Ittoop O, Blanchard S, and Furfine E. HIV protease inhibitors block adipogenesis and increase lipolysis in vitro. 6th Conference on Retroviruses and Opportunistic Infections, Chicago, Jan. 31 - Feb. 4 [abstract #666]. 2. Lenhard J, Weiel J, Paulik M, Lehmann J, Koszalka G, and Furfine E. Indinavir enhances retinoic acid signaling: Nelfinavir, saquinavir, and ritonavir inhibit retinoid effects in vitro. 6th Conference on Retroviruses and Opportunistic Infections, Chicago, Jan. 31 - Feb. 4 [abstract #665]. 3. Carr A, Samaras K, Chisholm DJ, and Cooper DA. Pathogenesis of HIV-1-protease-inhibitor-associated peripheral lipodystrophy, hyperlipidaemia, and insulin resistance. LANCET. June 20, 1998; volume 351 (9119), pages 1881-1883. New Antiviral Substances Found with HCG By John S. James HCG (human chorionic gonadotropin) is an approved drug which is prepared from the urine of pregnant women. Some commercial preparations have activity against HIV; however, it is believed that this antiretroviral activity is not due to the HCG itself, but due to other substances--impurities in the commercial product--which are sometimes associated with it. On March 16th a research team at New York University, Harvard Medical School, and the National Institutes of Health announced the purification and identification of three enzymes with anti-HIV activity from the urine of pregnant women, and also found related anti-HIV substances in human milk and some animal products.(1) Dr. Robert Gallo, of the Institute of Human Virology in Baltimore, has also been working on a separate project to identify one or more antiretroviral substances found in the urine of women in the first weeks of pregnancy(2) (see "New Approaches to HIV Treatment: Interview with Robert Gallo, M.D.," AIDS Treatment News #285, December 19, 1997). If these results are confirmed, they could lead to new strategies for developing antiretroviral drugs. References 1. Lee-Huang S, Huang PL, Sun Y and others. Lysozyme and RNases as anti-HIV components in beta-core preparations of human chorionic gonadotropin. Proceedings of the National Academy of Sciences, USA March 16, 1999; volume 96, issue 6, pages 2678-2681. 2. Lunardi-Isandar Y, Bryand JL, Blattner WA and others. Effects of a urinary factor from women in early pregnancy on HIV-1, SIV and associated disease. Nature Medicine, April 1998; volume 4, number 4, pages 428-434. Health GAP Coalition--New Group for International Treatment Access Our last issue (AIDS Treatment News #314) mentioned an "as- yet-unnamed effort" for broadening global access to essential treatments for AIDS and other diseases; on March 14 this new group was named the Health GAP (Global Access Project) Coalition.
 Health GAP Coalition is sending five U.S.
activists to the March 25-27 meeting in Geneva, Switzerland
on compulsory licensing of patents on essential medical
technologies (www.cptech.org/march99-cl), and is also
helping to mobilize support for the HOPE for Africa Act (see
"Africa Trade Legislation--April Lobby Day," below).
Health GAP Coalition is sending five U.S.
activists to the March 25-27 meeting in Geneva, Switzerland
on compulsory licensing of patents on essential medical
technologies (www.cptech.org/march99-cl), and is also
helping to mobilize support for the HOPE for Africa Act (see
"Africa Trade Legislation--April Lobby Day," below).
During the next year the Health GAP Coalition will mobilize around the World AIDS Conference (in Durban, South Africa, July 9-14, 2000) on issues of dramatically increasing worldwide access to AIDS treatments of all kind. This group was started by Alan Berkman, M.D., a New York physician who treats low-income and formerly homeless persons with AIDS and has worked internationally, with help from members of Doctors Without Borders, ACT UP/New York, ACT UP/Philadelphia, Search for a Cure, Ralph Nader's Consumer Project on Technology, AIDS TREATMENT NEWS, and others. Africa Trade Legislation--April Lobby Day The Health GAP Coalition is one of many organizations supporting the HOPE for Africa Act, introduced in Congress by Rep. Jesse Jackson, Jr. (D., Illinois) and 30 cosponsors. Among other provisions, this bill would prevent the U.S. government from threatening or pressuring sub-Saharan African countries to force them to do more to protect intellectual property than is required by GATT or other international treaties. Currently the U.S. is using such pressure on behalf of domestic and multinational pharmaceutical companies, denying lifesaving medical treatment to potentially millions of people in order to support corporate patent rights--often rights the companies do not even intend to use in those countries (see "Compulsory Licensing for Bridging the Gap-- Treatment Access in Developing Countries," AIDS Treatment News #314, March 5, 1999). Many supporters of the HOPE for Africa Act are opposing another Africa trade bill, the African Growth and Opportunity Act. An analysis of this bill by the South Centre--a Geneva- based organization which works with governments of developing countries--examines some good aspects and many more bad ones. The basic problem with the bill seems to be that U.S. commercial and ideological interests had nearly complete control of the agenda, and terms are to be dictated to African countries while their most important interests are ignored. A copy of the report, "Lopsided Rules of North-South Engagement: The African Growth and Opportunity Act," can be downloaded from www.cptech.org/ip/health/trade A community letter supporting the Jackson bill and opposing the other has been signed by over 30 AIDS organizations, including AIDS Action Council, National Minority AIDS Council, National Association of People with AIDS, Gay Men's Health Crisis, AIDS Project Los Angeles, and ACT UP/Golden Gate, ACT UP/New York, and ACT UP/Philadelphia, and given to all members of the House and Senate. The text and Congressional debates on both bills are available through the Library of Congress, at thomas.loc.gov (search for 'Africa'). A day of demonstrations and Congressional lobbying on these bills will be held in mid-April (the date was not set when this issue went to press). For more information about the Health GAP Coalition or the April lobby day, contact Asia Russell, asia@critpath.org, or through ACT UP/Philadelphia at 215-731-1844, or P.O. Box 22439, Land Title Station, Philadelphia PA 19110. Medical Marijuana: Major Report Released March 17 By John S. James
 The long-awaited Institute of Medicine report on medical
marijuana was released March 17. [Because this issue of AIDS
Treatment News went to the printer that morning, we could not
obtain the report by press time.]
The long-awaited Institute of Medicine report on medical
marijuana was released March 17. [Because this issue of AIDS
Treatment News went to the printer that morning, we could not
obtain the report by press time.]
Marijuana and Medicine: Assessing the Science Base should be available either at www2.nas.edu/medical-mj, or at National Academy Press, /www.nap.edu (click "Reading Room" to read or print a free copy, or "Bookstore" to purchase a copy, of this report or over 1,000 others). The Institute of Medicine (IOM) is part of the National Academy of Sciences, a prestigious scholarly organization chartered by Congress over a hundred years ago to advise the Federal government on scientific and technical matters. The IOM has conducted "an 18-month study to evaluate the therapeutic value of marijuana and its chemical components, particularly cannabinoids such as THC. The study will include an assessment of both the benefits and risks of marijuana and cannabinoids for human health." Our understanding is that the IOM was told not to make any recommendation on whether laws prohibiting medical use of marijuana should be changed. (According to Dale Gieringer of California NORML, the National Organization for the Reform of Marijuana Laws, California is currently spending more money to imprison medical marijuana offenders than to implement Proposition 215, the measure passed by the state's voters to allow medical use.) The IOM releases about two dozen such reports each year; other recent topics include quality of health care, preventing maternal transmission of HIV in the U.S., chemical and biological terrorism, lesbian health, and environmental justice. On March 15 the IOM was in the news with its report on the issue of whether or not the remaining stock of smallpox virus should be destroyed. California: 9th Annual AIDS Lobby Day, April 19 Each year major California AIDS groups organize a trip to Sacramento for people to meet their legislators on AIDS issues. This year's April 19 event will include morning briefings, a noon rally on the Capitol steps, and meetings legislators and staff in the afternoon. This event is organized by the California HIV Advocacy Coalition (CHAC), which includes AIDS Project Los Angeles, Project Inform, and the San Francisco AIDS Foundation. For more information, in Southern California call Greg Smith, 323-993-1366; in Northern California call Randy Allgaier, 415-487-3085. San Francisco Area: Important Cyclosporin Study Recruiting By John S. James An important study of possible interactions between cyclosporin and protease inhibitors is recruiting in San Francisco. Participants will stay 48 hours in the hospital for multiple blood draws, and be paid $300 after completing the study. Participants must have a CD4 count over 200, undetectable viral load, and be on stable treatment (no changes) that includes a protease inhibitor, for at least one month before entering the study. Cyclosporin is an immune-suppressive drug which under some conditions may inhibit HIV (although in recent years interest has shifted to cyclosporin derivatives which may be antiviral without being immune suppressive). This study is not considering treatment use, however, but only whether pharmacokinetic interaction between cyclosporin and protease inhibitors can change the blood levels of one or both of the drugs. It is important to have this information in order to overcome one of the obstacles which now make it very difficult for persons with HIV to receive organ transplants. Learning whether or not immune-suppressive drugs used after organ transplantation will interact with protease inhibitors, requiring dosage changes, would eliminate one of the uncertainties now used to argue against making organ transplants available. Volunteers will receive two doses of cyclosporin, one intravenously and one orally. As with any study there are risks, which are explained at length in the informed-consent document. For more information about this study, call Leslie Floren, Pharm.D., 415-502-4949. AIDS Treatment News Published twice monthly Subscription and Editorial Office: P.O. Box 411256 San Francisco, CA 94141 800/TREAT-1-2 toll-free U.S. and Canada 415/255-0588 regular office number Fax: 415/255-4659 E-mail: aidsnews@aidsnews.org
Editor and Publisher: John S. James Associate Editor: Tadd T. Tobias Reader Services: Tom Fontaine and Denny Smith Operations Manager: Danalan Richard Copeland Statement of Purpose: AIDS Treatment News reports on experimental and standard treatments, especially those available now. We interview physicians, scientists, other health professionals, and persons with AIDS or HIV; we also collect information from meetings and conferences, medical journals, and computer databases. Long-term survivors have usually tried many different treatments, and found combinations which work for them. AIDS Treatment News does not recommend particular therapies, but seeks to increase the options available. Subscription Information: Call 800/TREAT-1-2 Businesses, Institutions, Professionals: $270/year. Includes early delivery of an extra copy by email. Nonprofit organizations: $135/year. Includes early delivery of an extra copy by email. Individuals: $120/year, or $70 for six months. Special discount for persons with financial difficulties: $54/year, or $30 for six months. If you cannot afford a subscription, please write or call. Outside North, Central, or South America, add air mail postage: $20/year, $10 for six months. Back issues available. Fax subscriptions, bulk rates, and multiple subscriptions are available; contact our office for details. Please send U.S. funds: personal check or bank draft, international postal money order, or travelers checks. ISSN # 1052-4207 Copyright 1999 by John S. James. Permission granted for noncommercial reproduction, provided that our address and phone number are included if more than short quotations are used. |

© 1997-99 BEI