 |

|
| Treatment Highlights AIDS Treatment News #298 |
| Contents:
HIV-Specific Immune Responses Restored by HIV Immunogen Plus Antiretroviral Suppression Successful Treatment of "Buffalo Hump" with Growth Hormone Geneva Conference Treatment Highlights AIDS Treatment News Internet Directory, http://www.aidsnews.org Women's Treatment Information and Advocacy: Position at Project Inform San Francisco, Oakland: Treatment Access and Information Workshop, July 24, July 31, or August 14 Scientific American July Special Report on AIDS
HIV-Specific Immune Responses Restored by HIV Immunogen Plus Antiretroviral Suppression By John S. James
 In what might be the most important treatment presentation at the 12th World AIDS Conference (Geneva, June 28 - July 3), New York University immunologist Fred Valentine, M.D., reported a striking return of HIV-specific immune responses from a multicenter controlled trial of Remune (TM) (the Salk HIV treatment vaccine, being tested by the Immune Response Corporation of Carlsbad, California, and Agouron Pharmaceuticals, Inc., of La Jolla, California; see AIDS Treatment News #297)--in a 43-patient clinical trial where this potential treatment, called the HIV immunogen, was given to patients whose viral load had been very well suppressed by conventional antiretroviral treatment(1). In what might be the most important treatment presentation at the 12th World AIDS Conference (Geneva, June 28 - July 3), New York University immunologist Fred Valentine, M.D., reported a striking return of HIV-specific immune responses from a multicenter controlled trial of Remune (TM) (the Salk HIV treatment vaccine, being tested by the Immune Response Corporation of Carlsbad, California, and Agouron Pharmaceuticals, Inc., of La Jolla, California; see AIDS Treatment News #297)--in a 43-patient clinical trial where this potential treatment, called the HIV immunogen, was given to patients whose viral load had been very well suppressed by conventional antiretroviral treatment(1).
In almost all viral infections, control of the disease is associated with a very strong "lymphoproliferative response" (LPR)--the ability of certain immune-system cells in the blood to recognize the virus and then grow rapidly, producing many more such cells, which then mobilize other immune-system cells against the virus. With HIV infection, however, it has been known for years that the LPR to HIV is largely absent at all stages of disease--except for a small minority of patients, who do have a strong LPR against many antigens of HIV. These patients are always long-term nonprogressors, able to control the virus for many years and perhaps indefinitely, without treatment. Dr. Valentine presented preliminary results of a controlled, double-blind clinical trial at eight medical centers, which clearly showed that lymphoproliferative responses like those of long-term nonprogressors can be induced by treatment in patients who otherwise would not have them. These responses were seen against an unrelated strain of HIV (not only against the HIV immunogen itself). Whether these induced responses are helpful like those which develop naturally (earlier in infection) will not be known until some patients stop their antiretrovirals and see if, or how rapidly, their viral load returns.
Background The lymphoproliferative response, measured by a complex laboratory test, is a major indicator of how well a person's immune system responds to a particular antigen (an antigen usually consists of peptides, short sequences of proteins, produced by a particular virus, bacterium, or other disease- causing organism), which the body's cells had already learned to recognize through previous exposure. The lack of this response to HIV (in most patients, except for long-term nonprogressors) has been a central mystery of this disease. (Interestingly, chimpanzees--which can be infected with HIV but seldom become ill--do have a strong LPR against HIV.) The LPR is measured in the laboratory by culturing blood cells from the patient in the presence of the antigen of interest (here, HIV). Usually a small fraction of CD4+ T- helper cells will be able to recognize the particular antigen, and as a result they will begin to reproduce rapidly. The amount of cell growth in the whole cell culture can be measured by including tritium, a radioactive form of hydrogen, in the growth medium, in a form that will be picked up by and incorporated into growing cells. Later, technicians separate the cells from the remaining growth medium, and then they can easily measure how much radioactivity the cells have. The result of this test is given as a "stimulation index"—a measure of how well that person's immune-system cells respond to the antigen being tested. The stimulation index is the ratio of how much radioactivity is present in cells from a culture which included the antigen, vs. cells in a control culture which was treated the same except that the antigen was not added. For example, the stimulation index of an HIV-negative person who has been infected with CMV will often be in the range of five to 50--meaning that when their cells were exposed to the CMV antigen, a few recognized it and grew enough that the culture as a whole took up five times to 50 times as much tritium as the cell culture not exposed to the antigen. The stimulation index can be as large as 100 or more; or on the low end of the scale, a stimulation index of one means no response (and an index of two could also mean no response, due to errors in the testing). Measuring the LPR is labor intensive and requires highly skilled technicians in order to assure accuracy; as a result, this test of immune function has remained a research method and not become part of standard clinical practice. In patients with HIV (except for long-term nonprogressors) the LPR to HIV is very weak or not present at all. The LPR to CMV and other opportunistic pathogens with which the patient has been infected may be lost later in late-stage HIV disease--but if the HIV is then suppressed with antiretrovirals, the LPR to the opportunistic infections will often come back. But the LPR to HIV does not occur by itself, even when the virus has been fully suppressed and has remained undetectable for years. Why does the immune system of most patients fail to produce a lymphoproliferative response to HIV? No one knows for sure, but the dominant theory today is that HIV may kill off the small fraction of T-helper cells which are genetically able to recognize it--since these cells become activated during primary infection and therefore are vulnerable to being infected and destroyed by this virus. The lack of HIV-specific LPR might explain why viral load usually returns quickly if antiretroviral drugs are stopped. But in the few long-term nonprogressors, the body somehow gets the upper hand instead, HIV-specific responses are preserved, and the virus remains controlled without treatment. An excellent review of this area by Dr. Valentine and others appeared just before the Geneva conference in a supplement to the June issue of AIDS Research and Human Retroviruses(2). This article referred to the study which Dr. Valentine presented in Geneva (described below); however, the data was blinded when that publication went to press, meaning that the authors did not know what patients had received the real HIV immunogen and which had received a placebo. There was an indication that the treatment was working, however: "Although the study is still blinded, approximately one half of the subjects have developed stimulation indices to the gag protein of between 10 and 400... These stimulation indices and those obtained by immunization with envelope vaccines often are as large as those seen in long-term immunological nonprogressors who develop proliferative responses as a consequence of their initial encounter with the virus." [Note: The gag protein is a "core" protein of HIV. It tends to be more constant from strain to strain than the "envelope" proteins found on the surface of the virus.]
The Geneva Report In a 10-minute "late breaker" session, Dr. Valentine gave a preliminary report of a 32-week clinical trial conducted at eight research centers; this trial had been completed, but only the first 20 weeks of data had been analyzed and were available for the presentation. Forty three patients were all given antiretroviral therapy (indinavir plus AZT plus 3TC). Four weeks later, they were vaccinated with either the Remune HIV immunogen, or a control vaccination. The active or control immunization was repeated every three months. The purpose of this study was to see if the lymphoproliferative response could be induced by immunization when a patient's viral load was well suppressed by antiretroviral drugs; previous data had suggested that this might be possible. Other measurements included viral load (using an ultrasensitive test with a lower limit of 40 copies), CD4 counts, and the level of the chemokine MIP-1- beta. The Remune HIV immunogen--a vaccine-type treatment for persons already infected with HIV, designed by the late Dr. Jonas Salk and currently in a large phase III trial, is made from whole killed HIV from which the gp 120 envelope protein has been removed. It also contains an adjuvant (IFA, or incomplete Freund's adjuvant); an adjuvant is used to make a vaccine work better. The control vaccine used in this trial consisted of the adjuvant alone. According to a July 3 press release from New York University Medical Center, the 43 patients had a median CD4 count of 493 and a median viral load of 8,159 copies before they began the study. The patients who received the HIV immunogen consistently developed strong lymphoproliferative responses like those of long-term nonprogressors; some of the volunteers who received the immunogen had stimulation indices in the hundreds. Those who received the control vaccination (adjuvant only, without the killed HIV) had very low stimulation indices, usually around three for envelope proteins and slightly higher for gag. Dr. Valentine also noted data from long-term followup of one of the Merck trials; patients who had been on the same antiretroviral regimen and had their virus undetectable for almost three years (but who had not received any HIV immunization) had no spontaneous recovery of their lymphoproliferative response. The LPR was tested by using different antigens--not only from the HIV immunogen itself, but also a recombinant p24, and most importantly, a whole virus from clade B--a strain of HIV very different from the one used to make the immunogen. (Clade B is the form of HIV which is common in North America. But Dr. Salk developed the immunogen using an early HIV isolate from a woman in Zaire. This virus later was found to be a natural recombinant, consisting of a clade A envelope and a clade G gag protein. This cross-clade reactivity of the immunogen (to the clade B virus) suggests that if this treatment turns out to be useful, it could be applied to different HIV strains in different parts of the world. The immune response to a clade different from that of the antigen which induced it is unusual--but not especially surprising in this case, however, since this virus targets the gag protein, which tends to be relatively similar among different strains of HIV (the envelope differs much more, but this part of the virus is stripped off when the immunogen is made). The lymphoproliferative response recognizes peptides, which are short fragments of proteins--and the peptides from different viruses are often the same.
Comment This very important study leaves many questions unanswered. The most urgent is whether the patients who had lymphoproliferative responses induced in this way will benefit from it and perhaps become long-term nonprogressors. While it seems logical that the answer is yes, it is also possible that LPR is only a consequence or marker of a good immune response which controls the virus by some other mechanism; it might not be the cause of the viral control by long-term nonprogressors. Many scientists did not expect this treatment to work, since these patients had already been exposed to plenty of HIV antigens due to viral replication. A major question now is why the additional exposure to antigens through the vaccine was so much more effective. What surprises us is how well the study worked--since it can take months or years for naive cells to recover in an adult after immunosuppression. If the cells able to recognize HIV had indeed been wiped out, as the predominant theory suggests, could the lymphoproliferative responses have come back so strongly in only 16 weeks (from the four-week point of first immunization, to the 20-week point which contributed the last data available for presentation in Geneva)? If, however, the cells had not been destroyed but had been turned off or suppressed in some way, then it would be important to learn how the HAART (highly active antiretroviral treatment) plus immunogen reversed such an effect. Perhaps a more specific treatment could produce the same result. The volunteers in this trial were relatively early in HIV disease progression, but well past the stage of primary infection. No one knows how well the approach would work on persons with more advanced illness. It is often best to avoid the more difficult cases when first establishing a proof of principle--and then improve the treatment and extend it to more patients. Since this study was presented in one of two simultaneous late breaker sessions at the very end of the conference, there was little time for discussion among attendees before people went home. Part of the data (from the patients at the New York University site alone) had been reported by Dr. Valentine the day before, during a satellite session organized by the Immune Response Corporation; about 400 people showed up for this 6:30 a.m. meeting.
Other Studies A number of laboratory and animal studies relevant to restoring HIV-specific immunity were also presented at the 12th World AIDS Conference; we were not able to review them before going to press. Also, other human trials which may restore these responses are either ongoing, or now being organized.
References 1. Valentine FT, DeGruttola V, Kaplan M, and others. Effects of HAART compared to HAART plus an inactivated HIV immunogen on lymphocyte proliferative responses (LPR) to HIV antigens. 12th World AIDS Conference, Geneva, June 28 - July 3, 1998 [abstract # 31227 (late breaker session, #LB 9)]. 2. Valentine FT, Paolino A, Saito A, and Holzman RS. Lymphocyte-proliferative responses to HIV antigens as a potential measure of immunological reconstitution in HIV disease. AIDS RESEARCH AND HUMAN RETROVIRUSES June 1998; volume 14, supplement 2, pages S-161 to S-166.
Successful Treatment of "Buffalo Hump" with Growth Hormone By John S. James
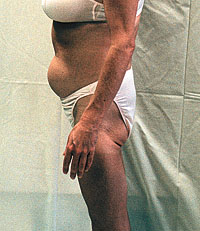 A poster at the 12th World AIDS Conference reported successful treatment of body fat redistribution in five patients(1). All showed improvement, from 25% to total resolution, usually with just a few weeks or months of the treatment; there was also improvement in truncal obesity in at least four of the patients. However, the patients' elevated cholesterol and triglyceride levels did not improve significantly. And there were suspected side effects of the therapy, including hyperglycemia in two cases, elevation of pancreatic enzymes in one, and carpal tunnel syndrome which led to discontinuation of treatment in another. A poster at the 12th World AIDS Conference reported successful treatment of body fat redistribution in five patients(1). All showed improvement, from 25% to total resolution, usually with just a few weeks or months of the treatment; there was also improvement in truncal obesity in at least four of the patients. However, the patients' elevated cholesterol and triglyceride levels did not improve significantly. And there were suspected side effects of the therapy, including hyperglycemia in two cases, elevation of pancreatic enzymes in one, and carpal tunnel syndrome which led to discontinuation of treatment in another.
The dose used was 5 to 6 mg per day of Serostim(TM), injected subcutaneously. This report was not from a study, but from experience in two physicians' practices. The treatment was given primarily for wasting, in the arms and legs--especially for the earliest patients, since it was not known that growth hormone would help correct the abnormal body fat redistribution. Recently we have heard anecdotally of similar cases of successful treatment of lipid redistribution with human growth hormone. This presentation in Geneva may be the first public report of successful treatment, other than surgery, for the body shape changes which seem to be associated with protease inhibitor treatment in some patients.
References Torres, RA and Unger KW. Treatment of dorsocervical fat pads (buffalo hump) and truncal obesity with Serostim(TM) (recombinant human growth hormone) in patients with AIDS maintained on HAART. 12th World AIDS Conference, Geneva, June 28 - July 3, 1998 [abstract # 32164]. Note: An updated and extended abstract was distributed at the conference, and is the one reviewed above.
Geneva Conference Treatment Highlights By John S. James
 About 12,000 people attended the 12th World AIDS Conference (Geneva, Switzerland, June 28 - July 3), the major international conference which takes place every two years. Unlike the previous meeting (Vancouver, July 7-12, 1996), where the big news was protease inhibitors, Geneva did not have one major story but was characterized by solid scientific advances--most of them good news. We are now in a period of incremental more than revolutionary treatment developments--although some of the reports in Geneva could be revolutionary if they are confirmed. About 12,000 people attended the 12th World AIDS Conference (Geneva, Switzerland, June 28 - July 3), the major international conference which takes place every two years. Unlike the previous meeting (Vancouver, July 7-12, 1996), where the big news was protease inhibitors, Geneva did not have one major story but was characterized by solid scientific advances--most of them good news. We are now in a period of incremental more than revolutionary treatment developments--although some of the reports in Geneva could be revolutionary if they are confirmed.
Perhaps the most important single change in atmosphere at this conference is that immunology is at last being taken more seriously than before. Of course it has always been known that the immune system is central to this disease, but it has been easier to find clear targets for treatment development against the HIV virus--and easier to identify indicators of antiretroviral activity (since clearly less virus is better than more) than to know which immune responses are beneficial. As a result, virology has dominated AIDS medical research, leaving AIDS immunology underfunded; the great majority of immunologists in the U.S. and the world today have never done any work in AIDS. Now there is widespread consensus that this disconnect between immunology and AIDS research must end. We left Geneva with more optimism than much of the mainstream press, which noted the lack of major treatment advances such as the protease inhibitors at Vancouver, some setbacks in treatments and vaccines, and the conclusion of many experts that in fighting the AIDS epidemic, the focus must still be on prevention, which is known to work but often held back by politics. This difference in outlook is not because either view is wrong, but because we are reporting different news. AIDS Treatment News is written primarily for persons who know they have HIV and who have at least some access to modern medical care (unfortunately the large majority of people in the world do not). There are many promising developments likely to result in important new treatment options during the next several months or years. And most of the bad news was already known, and has been widely reported in AIDS Treatment News and elsewhere. Certainly we agree on the importance of prevention; no one expects the epidemic to be stopped by finding and treating everybody, and no proven safe and effective vaccine is available today. But even though prevention is far more cost- effective than treatment in saving lives overall, this is no reason to give up on anybody. People need hope if they are to mobilize for effective policies; millions of lives may be saved by low-cost treatments; and research focusing on far less costly antiretrovirals can benefit everybody, even those with no financial constraints on their care.
We will also look at what is being done on the conference theme of "bridging the gap"--providing treatment for the 90 percent of people with HIV who do not have access today.
AIDS Treatment News Internet Directory http://www.aidsnews.org By Tadd Tobias AIDS Treatment News has rewritten its World Wide Web directory site to coincide with our poster presentation at the 12th World AIDS Conference in Geneva ("Internet AIDS Information--Providing Quality Referrals," abstract #34218). We developed this site to refer users to some of the Internet AIDS treatment information we consider most reliable and useful, and also to organize important "favorites" ("bookmarks") for convenient access by ourselves and others. We chose a simple design with text only, to avoid delay and software incompatibilities for users throughout the world. We link to major treatment sites, official treatment guidelines, news media stories, official conference sites, independent conference reports, conference and meeting calendars, advocacy and service organizations, medical reference books, medical journals, AIDS drug interaction guides, clinical trials information, AIDSLINE searches, "ask an expert" sites, pharmaceutical company sites, buyers' clubs for alternative/complementary therapies, and information on access to care, among other categories. To avoid repeating the work of others, we generally link to some of the most useful lists which are already being maintained elsewhere. Our official Web address is http://www.aidsnews.org. However, the 'www.' is optional for this site. And with most modern browsers, you only need to type 'aidsnews.org' (without the single quotes) to reach our Internet directory.
Women's Treatment Information and Advocacy: Position at Project Inform
 Project Inform, located in San Francisco, is hiring an Information and Advocacy Associate Coordinator, "to coordinate and develop Project Inform's advocacy efforts and treatment education materials on women-specific treatment issues (Project WISE) and treatments for a number of opportunistic infections." The projected hiring date is late July. Interested persons should send a resume, letter of interest, and samples of their writing to: IAA Search, Project Inform, 205 13th St. #2001, San Francisco, CA 94103. Project Inform, located in San Francisco, is hiring an Information and Advocacy Associate Coordinator, "to coordinate and develop Project Inform's advocacy efforts and treatment education materials on women-specific treatment issues (Project WISE) and treatments for a number of opportunistic infections." The projected hiring date is late July. Interested persons should send a resume, letter of interest, and samples of their writing to: IAA Search, Project Inform, 205 13th St. #2001, San Francisco, CA 94103.
The person hired will write treatment information for Project Inform publications, coordinate and participate in advocacy efforts for research relevant to women living with HIV, develop and implement strategies to improve access to promising treatments, and speak on behalf of the organization to community groups and others. Job requirements include knowledge of HIV-disease pathogenesis and treatment, knowledge of treatment issues of concern to women, written, telephone, and verbal communication skills, and computer experience.
San Francisco, Oakland: Treatment Access and Information Workshop, July 24, July 31, or August 14 A free, one-afternoon workshop for case managers, counselors, social workers, HIV service providers, and treatment advocates will provide training in how to help clients gain access to experimental or expensive treatments, and to find HIV information and resources on the Internet and elsewhere. This workshop, sponsored by the Community Consortium (an organization of HIV physicians in the San Francisco area) will be given in Oakland (Friday July 24, noon to 3:30 p.m., at the Center for AIDS Services, 5720 Shattuck Avenue at 57th St.), and in San Francisco (Friday July 31, and also Friday August 14, noon to 3:30 p.m., at the University of California San Francisco Mission Center Building, 1855 Folsom Street at 15th.) "We will give you an update on clinical issues that are relevant to your clients, especially regarding side effects and drug interactions with illegal or recreational drugs. Second, we will teach you how to get your client access to care; we'll show you how to link clients to clinical research, drug assistance programs, and physician referral programs. Finally, we will teach you how to keep yourself-- and your clients--educated about HIV treatment issues in the future." Although there is no fee, space is limited and preregistration is requested. To register, fax your name, agency, address, phone number, and the date you are interested in attending to 415-476-6948, or call the Community Consortium at 415-502-0657.
Scientific American July Special Report on AIDS The July issue of Scientific American, now on the newsstands, includes an excellent 10-article special report on AIDS, written by leading experts in treatment, prevention, epidemiology, vaccines, and other topics. Most of our readers already have this information, but may find the issue useful for friends, colleagues, or others who have not been involved with AIDS and want current background on the epidemic.
AIDS Treatment News Published twice monthly Subscription and Editorial Office: P.O. Box 411256 San Francisco, CA 94141 800/TREAT-1-2 toll-free U.S. and Canada 415/255-0588 regular office number Fax: 415/255-4659 E-mail: aidsnews@aidsnews.org
Editor and Publisher: John S. James Associate Editor: Tadd T. Tobias Reader Services: Tom Fontaine and Denny Smith Operations Manager: Danalan Richard Copeland Statement of Purpose: AIDS Treatment News reports on experimental and standard treatments, especially those available now. We interview physicians, scientists, other health professionals, and persons with AIDS or HIV; we also collect information from meetings and conferences, medical journals, and computer databases. Long-term survivors have usually tried many different treatments, and found combinations which work for them. AIDS Treatment News does not recommend particular therapies, but seeks to increase the options available. Subscription Information: Call 800/TREAT-1-2 Businesses, Institutions, Professionals: $270/year. Includes early delivery of an extra copy by email. Nonprofit organizations: $135/year. Includes early delivery of an extra copy by email. Individuals: $120/year, or $70 for six months. Special discount for persons with financial difficulties: $54/year, or $30 for six months. If you cannot afford a subscription, please write or call. Outside North, Central, or South America, add air mail postage: $20/year, $10 for six months. Back issues available. Fax subscriptions, bulk rates, and multiple subscriptions are available; contact our office for details. Please send U.S. funds: personal check or bank draft, international postal money order, or travelers checks. ISSN # 1052-4207 Copyright 1998 by John S. James. Permission granted for noncommercial reproduction, provided that our address and phone number are included if more than short quotations are used.
|
© 1997-98 BEI