 |

|
| |
|
Contents:
Lipodystrophy Research Coordination Needed PMPA Trials Recruiting Improved Viral Load Test: Open Letter on FDA Approval Titillationism--Or, Register Now to Vote! By John S. James Lipodystrophy Research Coordination Needed By John S. James
Treatment activists are increasingly concerned that the research needed to understand and control lipodystrophy is not being coordinated properly, with some of it not done at all. Patients and advocates have certain advantages in calling attention to these problems, as they can speak more freely than corporate or government officials, or even academic researchers who may need to cultivate relationships to maintain funding. Activists are less constrained by organizational interests and positions, or professional territories they have staked out. In this article we did not quote individuals, because the overlap and consensus of many ideas would make implied authorship misleading. Here are some of the problems we have heard repeatedly, and suggestions for improvement: Lack of Research on Mechanisms. If medical researchers knew what caused these problems, how they arose and developed, it might be easy to find effective remedies. Theories have been proposed, but the only widespread consensus today is that no one much knows what is going on. This situation calls for consulting with leading researchers in many areas (including those who have not worked in AIDS before), to find plausible leads for potential mechanisms, and ideas for feasible, productive research. Someone in a clinic or lab may already have a lead which could be a major advance, but for various reasons, including commitment to other projects, they cannot develop it. There needs to be a coordinated program which can receive, evaluate, and prioritize these ideas, and act on them when appropriate. Even in the unlikely event that no good research leads are available, it is still possible to do empirical research--for example, by running hundreds of commercial and research tests on a few patients and a few normal controls, looking for unexpected differences which might suggest new leads for further investigation. Research to find mechanisms does not necessarily have to wait for an agreed definition of the problem. Intensive laboratory testing could be done with only a few similar patients--or even only one patient. Successful understanding and treatment of one individual would almost certainly produce knowledge which could also be used for many others, although not for all. Flight of the Pharmaceutical Companies. Again and again we are hearing from activists that pharmaceutical companies are not funding studies of problems with their drugs, because they do not want to know about or raise the profile of these issues. The mechanism research noted above, which might bring new problems to light, may be particularly disfavored; companies can have some incentive to support a clinical trial which could show that their product causes less problem than a competitor's. The adverse effects associated with use of protease inhibitors were not expected, not sudden and dramatic, and slow to be recognized. Persons and organizations alike often first respond to this kind of problem by trying to ignore it away--which can create a corporate culture of denial. No one wants to be the messenger who brings attention to their organization's problems. What can be done to get corporations to take more responsibility for serious safety issues with their products? One approach is to build consensus among medical professionals, corporate scientists, the FDA, academics, and the patient community about what research is needed. If this does not work, we may need to consider the legal responsibility of a corporation if it does not research well- founded safety concerns which have been brought to its attention. Task Force Needed for Coordination and Leadership in Government and Elsewhere. Activists agree on the need for a task force which would include government agencies (FDA, NIH, and perhaps CDC and others), industry scientists, physicians and researchers in academia and elsewhere, and patient advocates. This group should include members or consultants who have critical expertise in metabolic or other fields, even if they are not familiar with HIV and AIDS. Need for a Definition. Lack of a generally accepted definition of the problem has made it hard to compare the work of different research groups--leading, for example, to very different findings on how often these problems occur. Better Use of Existing Data. Automated databases of patient information, cohort studies, registries of patients with certain symptoms, and data from clinical trials which were run for other purposes, should be considered as a relatively inexpensive kind of research, since it does not require conducting new trials. Activists realize, however, that much of this data has serious limitations. Important blood work may not have been collected--especially since many of the laboratory tests needed for studying lipodystrophy are not reliable unless the blood is drawn while fasting, and it is often difficult to get study volunteers to schedule morning visits because of interference with their jobs. Planning and Research Already Underway Probably the most advanced effort to plan and conduct research to address the lipodystrophy problem is within the AIDS Clinical Trials Group (ACTG), run by the U.S. National Institute of Allergy and Infectious Diseases. The ACTG cannot address all of the research needs, however, because it specializes in running large clinical trials and collecting high-quality data, but is not well organized to conduct the basic-plus-applied research to understand the biochemical mechanisms underlying a clinical problem (although the data it generates will eventually be useful for this purpose). The ACTG is planning a major study, called ACTG 384, primarily to compare certain treatment combinations including nelfinavir (a protease inhibitor) vs. regimens including efavirenz (a non-nucleoside reverse transcript inhibitor) vs. regimens with both; this complex study will run for up to three years. Included in ACTG 384 is a metabolism substudy, called A5005s, which "is designed to evaluate changes in glucose regulation, lipid metabolism, and body fat distribution in a subset of 345 subjects" (quoted from the protocol). The substudy will measure "insulin resistance, fasting lipid profiles, cytokines, cortisol, sex hormones, and body composition by measurements of weight and BIA at baseline and periodically during the study; in addition, oral glucose tolerance testing and regional body composition by DEXA scanning will be performed at a limited number of sites" (quoted from Metabolic Consequences Of HIV Disease and Treatment: An Overview of Current Knowledge and Research, Forum for Collaborative HIV Research, June 1998). Since some volunteers will be switched onto or off of protease inhibitors (due to the failure of their initial regimen, whichever it was), resulting changes in metabolic parameters can be followed. But since this study is mostly blinded and lasts for three years, the information will not be available soon. Another group, the Forum for Collaborative HIV Research, an independent public-private partnership to facilitate discussion and collaboration in HIV treatment research, is planning a meeting to bring together all the pharmaceutical companies working in antiretroviral drugs, clinical researchers in metabolic and gastrointestinal complications of HIV, patient advocates, physicians and other healthcare providers, and representatives from government agencies. This meeting's purpose is to discuss the design, implementation, and funding of a study to determine the incidence of metabolic complications (which patients are getting what problems)--what this study should look like, how many people it should measure, how long to follow them, where to place the study, and how to get it paid for. The reason for the focus on incidence of the symptoms is to provide the data necessary to develop a comprehensive definition of lipodystrophy. Some treatment activists believe that neither of these efforts will address the whole problem. ACTG 384 will produce useful data eventually, from a handful of specific drug regimens; its main focus is to compare a protease-inhibitor regimen with a non-protease-inhibitor regimen, not to find either the causes or treatments of lipodystrophy. The Forum for Collaborative HIV Research is also not focused on causes or treatment, but on preliminary steps of studying incidence in order to create a well-informed definition of the problem. Meanwhile, independent medical groups and researchers are reporting their data. At the ICAAC conference later this month (Interscience Conference on Antimicrobial Agents and Chemotherapy, September 24-27 in San Diego), one oral session titled "Metabolic Effects of Protease Inhibitors" will include about 10 different presentations; a separate poster session, "Toxicity and Metabolic Effects of Antiretroviral Therapy," will have almost 20. Comment Our provisional analysis is that the most serious missing piece in the research picture today is the lack of small, laboratory-intensive studies designed to elucidate the biochemical mechanisms of lipodystrophy. As noted above, mechanism studies do not need to wait for a definition. And the ACTG corporate culture of large, multicenter trials does not encourage the flexibility to move rapidly in changing research designs in response to new information--although of course the trials can generate crucial supporting data. The pharmaceutical companies seem best equipped to do these studies for their own drugs, but the legal system may be creating perverse incentives to ignore the problem instead. The law seldom requires a company to conduct or fund any particular study. But if the research does happen and a problem is discovered, the company may have to do something to avoid lawsuits later--even though there may not be anything to do immediately (other than pulling the drug off the market, which nobody would want unless the problem is very serious). Could an implicit strategy which amounts to legal protection through ignorance be behind the reluctance to investigate lipodystrophy problems expeditiously? Electronic Mailing Lists for More Information
LIPIDLIST: LIPIDLIST is sponsored by ACT UP/Golden Gate in San Francisco; it originated from a public forum held at the 12th World AIDS Conference in Geneva. Subscribers usually receive one or two messages a day. To subscribe to LIPIDLIST, send an email message to: listproc@critpath.org. The first line of the message should be:
subscribe lipidlist
where
CRIX-LIST:
CRIX-LIST currently transmits about 15 messages a day. To avoid getting too many email messages, you may want to subscribe to the Digest version, which combines each day's messages into a single transmission. CRIX-LIST has been archived back to January 1997, so you can obtain the earlier messages if you want. Instructions for subscribing to and using CRIX-LIST are at http://crix.pinkpage.com. Information about other AIDS-related email lists is available on the HIV InfoWeb site; see http://www.infoweb.org/treatment/index.html. PMPA Trials Recruiting:
A phase II trial of an oral prodrug of PMPA, an experimental antiretroviral, began enrolling September 4 and is seeking 175 patients at research sites in 20 U.S. cities. And a separate phase I/II study of oral PMPA plus hydroxyurea is enrolling in three cities: Baltimore, San Francisco, and Seattle. Both trials are being conducted by Gilead Sciences, headquartered in Foster City, California. (Baltimore and Seattle are listed in parentheses above because only the combination trial is located there; San Francisco has both (its combination study with hydroxyurea is currently full, although more volunteers may be sought later.) The larger trial (not involving hydroxyurea) is seeking volunteers who have a viral load between 400 and 50,000 copies, and who have been on a stable antiviral regimen for at least eight weeks; there is no CD4 count requirement. Volunteers will be randomly assigned to receive one of three doses of PMPA, or a placebo, in addition to maintaining their ongoing regimen. The trial will last 48 weeks, and after 24 weeks, those who had been assigned to placebo will be eligible to switch to the active drug. The oral form of PMPA, which is used in this trial, is usually taken once per day. The smaller trial of the combination of PMPA plus hydroxyurea requires a viral load of at least 10,000 copies, and a CD4 count of at least 200. Hydroxyurea has been shown to enhance the activity of PMPA in laboratory studies. Comment PMPA is currently one of the more promising experimental anti-HIV drugs in the pipeline. It is chemically related to PMEA, which is the active form of adefovir dipivoxil (PREVEON(TM)), which is currently available on expanded access. But laboratory, animal, and human studies of up to 28 days of dosing have suggested that PMPA may be the more effective drug--if it is found to be safe when used for a long time. (A full report on the 28-day human study is appearing now in ANTIMICROBIAL AGENTS AND CHEMOTHERAPY; we have not seen that article before going to press. An earlier account of the results was presented at the Retroviruses conference in February of this year.) A caution about the PMPA trial is that the related drug adefovir can cause kidney toxicity in long-term use. PMPA might or might not be similar; if it is, this toxicity could develop after several months, and would not have been seen in the 28-day trial. Therefore it is important that volunteers be monitored carefully, and not discount any required safety tests as merely routine. Note: Both PMEA and PMPA are not used directly, as they would have to be given by injection. Instead, for each of them a once-a-day "prodrug" has been prepared; these are related compounds which are taken orally and are chemically changed into PMEA or PMPA by the body. These oral drugs are bis-pom PMEA (also called adefovir dipivoxil, or PREVEON), and bis- poc PMPA (which is being used in the trials described here). For More Information For more information about either the 20-city PMPA trial, or the 3-city PMPA-plus-hydroxyurea trial, call either the AIDS Clinical Trials Information Service, 800-TRIALS-A, or Gilead Sciences Medical Information, 800-GILEAD-5. Both organizations should soon be ready to provide information about these trials, or refer callers to a site in one of the cities listed above. Improved Viral Load Test: Open Letter on FDA Approval A community consensus letter to the FDA is asking the Agency to move quickly on the application for approval of the UltraSensitive HIV viral load test, developed by Roche Molecular Systems. The UltraSensitive test uses a small modification to the standard viral load test which is already approved, to get reliable data as low as 50 copies, instead of the current 400-copy limit. Many doctors are already using the new version, because a value below 50 is a better indication that control of the virus can be maintained with the patient's current drug regimen. But there are often serious difficulties in getting the new test paid for, especially by public programs like Medicaid and Medicare, because it is not officially approved. Much work is now being done to allow access in major public hospitals in the New York City area. But the problem is national. The consensus letter has already been signed by treatment experts at ACT UP/Golden Gate, ACT UP/New York, AIDS Project Los Angeles, Gay Men's Health Crisis, National AIDS Treatment Advocacy Project, National Minority AIDS Council, San Francisco AIDS Foundation, Seattle Treatment Education Project (STEP), Test Positive Aware Network, and other organizations. To get a copy of the letter, find out how to sign on, or find out how to contact the FDA directly about this issue, check the Web site of the National AIDS Treatment Advocacy Project, http://www.natap.org. Titillationism--Or, Register Now to Vote! By John S. James
All major elections are critical this time because a new and ugly sexual inquisition could grow from the Starr-Clinton- Lewinsky national psychodrama. If an extramarital affair or its coverup disqualifies one from public office, what about a gay relationship? "Traditional values" regarded homosexuality in any form or context as considerably worse than a heterosexual affair. Stigma around AIDS is still widespread (as recently illustrated by a 570% increase in sales of the home HIV test kit when Longs Drug Stores moved it from a locked glass case to an open shelf, where customers did not have to ask the pharmacist for the test). A national sex panic would harm individuals affected, and also impede efforts to prevent new infections. We coined the word "titillationism" to mean an unholy alliance between prudery and prurience--illustrated here by the semen-stained dress, oral sex reports on television news, the search for "truth" meaning sexual details, and most graphically the 445-page Starr report. 20 million copies were delivered by Internet within two days of its publication; without the sex, a massive legal/political document in the news--dumped onto a public already fed up with the issue-- would be lucky to get one percent of that audience. We may be fortunate so far that the attacks on Clinton have focused on lying more than on sex. But the lying issue has its own disconnect, since the nation and the world would clearly be better off if the Clinton/Lewinsky relationship had remained private. As people reflect that in this case the "truth" was not in their interest--dredged up at great expense, for no public purpose, and despite the risk of damaging the efforts to prevent a worldwide economic catastrophe--the inquisitors may shift their focus back to sex. Analysis of supermarket tabloid articles (on any issue) shows a curious imbalance, with *personal* failings, flaws, mistakes, or other wrongness of an individual being the dramatic focus of many stories, while *institutional* failings almost never appear. In the mainstream press (such as the leading big-city daily newspapers, and TV network news or news magazines), this focus is usually more balanced, with both individual and institutional flaws reported. But with Clinton/Lewinsky the mainstream press has switched to tabloid style, focusing overwhelmingly on the personal (whether Clinton is telling the truth, for example) over the institutional (such as whether the national press--led in this case by The New York Times--is subordinating national interest to partisan agendas). The November 3 elections could bring to power officeholders who owe their success to a "sexual McCarthyism," a new meanness in U.S. politics. The danger is that many people will stay home on election day because they are turned off by the recent events, allowing an intolerant minority to dictate the outcome. By voting and encouraging others to do so, you can help stop that from happening. AIDS Treatment News Published twice monthly Subscription and Editorial Office: P.O. Box 411256 San Francisco, CA 94141 800/TREAT-1-2 toll-free U.S. and Canada 415/255-0588 regular office number Fax: 415/255-4659 E-mail: aidsnews@aidsnews.org
Editor and Publisher: John S. James Associate Editor: Tadd T. Tobias Reader Services: Tom Fontaine and Denny Smith Operations Manager: Danalan Richard Copeland Statement of Purpose: AIDS Treatment News reports on experimental and standard treatments, especially those available now. We interview physicians, scientists, other health professionals, and persons with AIDS or HIV; we also collect information from meetings and conferences, medical journals, and computer databases. Long-term survivors have usually tried many different treatments, and found combinations which work for them. AIDS Treatment News does not recommend particular therapies, but seeks to increase the options available. Subscription Information: Call 800/TREAT-1-2 Businesses, Institutions, Professionals: $270/year. Includes early delivery of an extra copy by email. Nonprofit organizations: $135/year. Includes early delivery of an extra copy by email. Individuals: $120/year, or $70 for six months. Special discount for persons with financial difficulties: $54/year, or $30 for six months. If you cannot afford a subscription, please write or call. Outside North, Central, or South America, add air mail postage: $20/year, $10 for six months. Back issues available. Fax subscriptions, bulk rates, and multiple subscriptions are available; contact our office for details. Please send U.S. funds: personal check or bank draft, international postal money order, or travelers checks. ISSN # 1052-4207 Copyright 1998 by John S. James. Permission granted for noncommercial reproduction, provided that our address and phone number are included if more than short quotations are used. |
© 1997-98 BEI
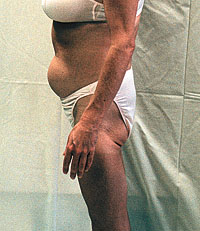 The word 'lipodystrophy', technically defined as "defective
metabolism of fat," has come to refer to a group of symptoms
seen increasingly with the use of protease inhibitors--
including body fat redistribution ("protease paunch,"
"buffalo hump," and other body-shape changes), high
triglycerides, high cholesterol, increase in blood glucose
with some cases of diabetes, and other adverse events. Many
experts suspect that these problems are not all part of a
single syndrome, but probably result from more than one
cause.
The word 'lipodystrophy', technically defined as "defective
metabolism of fat," has come to refer to a group of symptoms
seen increasingly with the use of protease inhibitors--
including body fat redistribution ("protease paunch,"
"buffalo hump," and other body-shape changes), high
triglycerides, high cholesterol, increase in blood glucose
with some cases of diabetes, and other adverse events. Many
experts suspect that these problems are not all part of a
single syndrome, but probably result from more than one
cause.
 The most direct and current information about lipodystrophy
is on Internet email lists, where patients discuss their own
experiences and treatments, and research findings as well.
Two of these lists are particularly relevant:
The most direct and current information about lipodystrophy
is on Internet email lists, where patients discuss their own
experiences and treatments, and research findings as well.
Two of these lists are particularly relevant:
 Atlanta, (Baltimore), Berkeley, Birmingham, Brookline,
Chicago, Cleveland, Dallas, Denver, Hershey, Houston, Los
Angeles, Minneapolis, New York City, Phoenix, Pittsburgh,
Portland OR, Providence, San Diego, San Francisco, (Seattle),
Tampa
Atlanta, (Baltimore), Berkeley, Birmingham, Brookline,
Chicago, Cleveland, Dallas, Denver, Hershey, Houston, Los
Angeles, Minneapolis, New York City, Phoenix, Pittsburgh,
Portland OR, Providence, San Diego, San Francisco, (Seattle),
Tampa
 Major national elections are November 3, but you must
register *now*, by early October, to be able to vote. You
might need to re-register if you have moved, or if you did
not vote in the last election; call your Registrar of Voters
to check the requirements in your area. You can vote
absentee, or by mail, but in order to do so you must not only
be registered, but also apply in time. Pundits have predicted
a low turnout--which means that your vote, and the friends
you get to the polls, will count more. Doug Ireland's article
in POZ magazine, October 98, page 47, listed Senate and House
races which may be particularly important for people with
AIDS or HIV.
Major national elections are November 3, but you must
register *now*, by early October, to be able to vote. You
might need to re-register if you have moved, or if you did
not vote in the last election; call your Registrar of Voters
to check the requirements in your area. You can vote
absentee, or by mail, but in order to do so you must not only
be registered, but also apply in time. Pundits have predicted
a low turnout--which means that your vote, and the friends
you get to the polls, will count more. Doug Ireland's article
in POZ magazine, October 98, page 47, listed Senate and House
races which may be particularly important for people with
AIDS or HIV.