 |

|
| |
|
Contents:
Efavirenz (SUSTIVA(TM)) Approved Crixivan(R) (Indinavir): Three Doses Daily, Not Two, in Most Regimens Amprenavir (Agenerase(TM)) Now Available in Expanded Access Successful Standard Treatment for Lipid Abnormalities: Experience with 44 Patients San Francisco: Body Shape Changes and Lipodystrophy-- Community Forum October 19 ICAAC Conference: Daily Summaries Available Abacavir (Ziagen(TM)) Advisory Committee Meeting, November 2 Prison Treatment Programs: Glaxo-Wellcome Awards 19 Grants Iscador Update: Interview with Robert Gorter, M.D. United States Conference on AIDS, Dallas, Oct. 29 - Nov. 1 San Francisco: POZ Life Expo, October 16-17
Efavirenz (SUSTIVA(TM)) Approved
By John S. James
 On September 18 DuPont Pharmaceuticals announced that its
antiretroviral efavirenz (brand name SUSTIVA) had been
approved by the FDA. Efavirenz had already been available
through a large expanded-access program (over 14,000 people
worldwide), but only for patients who could not be treated
effectively with approved drugs. Now that it has been
approved "for the treatment of HIV-1 infection"--meaning that
the FDA has not recommended its use be limited to any
particular group of patients--the medical community will need
to make the more difficult decisions on how to use this drug
with patients who have many treatment options. On September 18 DuPont Pharmaceuticals announced that its
antiretroviral efavirenz (brand name SUSTIVA) had been
approved by the FDA. Efavirenz had already been available
through a large expanded-access program (over 14,000 people
worldwide), but only for patients who could not be treated
effectively with approved drugs. Now that it has been
approved "for the treatment of HIV-1 infection"--meaning that
the FDA has not recommended its use be limited to any
particular group of patients--the medical community will need
to make the more difficult decisions on how to use this drug
with patients who have many treatment options.
Efavirenz has been approved for children as well as for adults. Efavirenz is a "non-nucleoside reverse transcriptase inhibitor"--sometimes abbreviated NNRTI; the other approved drugs in this class are nevirapine (Viramune(R)), and delavirdine (Rescriptor(R)). These drugs target the reverse transcriptase enzyme of HIV-1; but unlike AZT, ddI, etc., which also target this enzyme, the NNRTIs are not nucleoside analogs, as they do not provide "false building blocks" for the DNA created by the virus. All of the approved NNRTIs are not active against HIV-2, a variant of HIV-1 which is found in some parts of Africa. Viral resistance to NNRTIs can develop rapidly, so these drugs must be used carefully, in combination with other antiretrovirals to which the person has not been previously exposed, so that HIV replication is kept at very low levels, to give less opportunity for resistant virus to develop. Because of cross-resistance within the NNRTI class, once significant viral resistance has occurred to one of these drugs, the other two are also probably ineffective for that patient. A major question now is whether or not the medical community will be comfortable using efavirenz plus two nucleoside analogs, without a protease inhibitor, when beginning 3-drug treatment (and for which patients?). Or will physicians and patients stay with the now-standard three-drug regimens (protease inhibitor plus two nucleosides), or start with four drugs (protease inhibitor plus efavirenz plus two nucleoside analogs)? These complex decisions involve not only the long- term antiretroviral activity vs. toxicities of the initial drug combination, but also long-term treatment strategy: what drug combinations will be available for later use if the first treatment fails? At this time it is too early to know what medical consensus will develop. One example of the complexity concerns the consequences of virological treatment "failure." A major trial compared efavirenz plus AZT plus 3TC (lamivudine, brand name Epivir(R)--often combined with AZT in a single pill called Combivir(R)) vs. a standard-of-care regimen (indinavir--brand name Crixivan(R)--plus AZT plus 3TC), in patients who had already been treated with nucleoside analogs except 3TC but had not taken any protease inhibitors or NNRTIs; the efavirenz-containing regimen did at least as well as the indinavir-containing regimen at 24 and 36 weeks, as measured by the proportion of patients whose viral load remained under the 400-copy limit of the test (the same result was also found with a 50-copy limit). (See the FDA-approved "package insert," which summarizes some of the 24-week data; the recent ICAAC conference included data to 36 weeks with similar results.) But even in the efavirenz arm, at 24 weeks fewer than 80% of those originally assigned to the regimen still had a viral load under 400 copies (by the most rigorous method of counting, which considers anyone who dropped out of the study for any reason, or was missing important data, to be a treatment failure). What happens to the patients, on either regimen, who fail to keep the virus under control? With protease inhibitors there is experience indicating that those who "fail" treatment virologically (in that the viral load becomes measurable again), but who stay on the drugs, still seem to benefit, in that CD4 counts tend to stay higher, and there are fewer AIDS-related illnesses and deaths, than would have been expected for those patients. No one knows why this seems to be the case; possibly the virus which has become resistant to protease inhibitors has become less damaging in some way. But with efavirenz, there is not enough experience yet to know if there could be any similar benefit even after virologic failure occurs. A major hope for efavirenz is that it may reduce the need for protease inhibitors and therefore reduce the lipodystrophy problems which have become increasingly serious. But at this time no one knows for sure that protease inhibitors are causing these problems, or that efavirenz will not do so in long-term use. Considerations on Taking Efavirenz (SUSTIVA)
Also, the company has set up a phone line (800-4PHARMA), allowing callers to question an information specialist (in English or in many other languages, using an AT&T translation service). Persons can also request a package insert or patient package insert by fax or by mail. (For mailed information a full name is required, since to maintain confidentiality, the company will not mail a package which may be opened by the wrong person.) Efavirenz Pricing Controversy Well over 100 organizations and 200 individuals have signed a consensus statement protesting the price of Sustiva, which was set well above the price of the other drugs in the NNRTI class. [Note: This writer is a member of the Fair Price Working Group, which circulated the statement.] A particular concern is that this price will delay acceptance by many state ADAPs (AIDS Drug Assistance Programs), which will have to balance efavirenz against other important drugs which they will no longer be able to provide, due to the additional expense. As we go to press, negotiations are focusing on providing relief to the ADAPs. (Medicaid and the Veterans Administration already have low drug prices; and due to the expense of AIDS drugs, few individuals can pay for them out of pocket.) We will report on the pricing situation as it develops. You can contact the Fair Price Working Group by email to linda_grinberg@prodigy.com, or by fax at 310-471-4565. Reimbursement Assistance, and Expanded Access Contacts DuPont Pharmaceuticals has assured the community that it will not deny access to Sustiva if somebody needs the drug. Persons who need help getting the drug paid for can call 1- 800-334-4486, for reimbursement counseling to advise patients on public or private programs under which they may be eligible. There is also a patient assistance program to provide drug without charge to patients who meet certain financial qualifications and have no other way to have the drug paid for. Persons on expanded access when Sustiva is approved will be given a one-month supply to allow time to make arrangements to pay for the drug. According to DuPont Pharma, no one on expanded access will be denied the drug, even if they have no way to pay and fail to meet the financial criteria for the patient assistance program. If they are initially turned down for the patient assistance program, they can appeal, and all these appeals will be granted. A letter explaining the transition from the expanded access program will be sent to the investigators for delivery to patients at their next visit. Approval is pending in Canada and Europe; meanwhile, the expanded access programs there will soon begin to enroll children. For more information, patients in Canada can call 800-998-6854, or in Europe, call +44 (0) 38 842779. Note: Efavirenz will be marketed as Sustiva only in the U.S., Canada, UK, Spain, France, Germany, and Italy. Elsewhere Merck will market efavirenz under the brand name Stocrin(TM). Apparently the companies could not agree on a price for the rights elsewhere. Crixivan(R) (Indinavir): Three Doses Daily, Not Two, in Most Regimens By John S. James
 On September 18 Merck & Co., Inc. announced that it had
discontinued clinical trials with twice-daily dosing of
indinavir (Crixivan) in combination with nucleoside analogs
only. However, it is still continuing studies of twice-daily
indinavir dosing in combination with protease inhibitors
nelfinavir or ritonavir (which increase the time that
indinavir stays in the bloodstream). The FDA-approved dosing
of indinavir is every eight hours, but many patients had
switched to twice daily after an earlier small trial had
suggested that the two regimens might be equivalent. On September 18 Merck & Co., Inc. announced that it had
discontinued clinical trials with twice-daily dosing of
indinavir (Crixivan) in combination with nucleoside analogs
only. However, it is still continuing studies of twice-daily
indinavir dosing in combination with protease inhibitors
nelfinavir or ritonavir (which increase the time that
indinavir stays in the bloodstream). The FDA-approved dosing
of indinavir is every eight hours, but many patients had
switched to twice daily after an earlier small trial had
suggested that the two regimens might be equivalent.
The twice-daily dosing was discontinued after a new larger trial found that at 24 weeks, 91% of those on the approved three-times dosing regimen had a viral load below 400 copies, compared to only 64% of those with twice-daily dosing. A total of 87 patients had reached the 24-week time point and were included in this comparison. Besides the indinavir, participants in both treatment arms were taking AZT plus 3TC. They had not been treated with protease inhibitors, or with 3TC, before beginning this study. Discontinuation rates were low and similar in both groups. Also, there was a trend toward more discontinuation of the twice-daily regimen due to nausea and vomiting. Persons using indinavir twice daily should promptly check with their physicians about options for modifying their treatment. Amprenavir (Agenerase(TM)) Now Available in Expanded Access On September 21 both Glaxo Wellcome and Vertex Pharmaceuticals Inc. announced an expanded-access program for the experimental protease inhibitor amprenavir (brand name Agenerase, formerly known as VX-478 or 141W94). This program will be open to patients failing treatment with currently approved protease inhibitors. Glaxo and Vertex are working together on the development of amprenavir; Vertex discovered the drug, and Glaxo is responsible for manufacture, clinical trials, and submission for FDA approval. Here is the rapidly-changing information on this program, as of September 30 when this issue went to press. There are three different open-label protocols in the expanded-access program: Protocol 30010 is for patients who either had viral rebound or were intolerant on a currently-approved protease inhibitor therapy. This protocol is open to both adults and children (ages 4 and above). Protocol 30011 is for those who are intolerant to their current protease-inhibitor therapy; they can be changed to a specific amprenavir-plus-abacavir protocol, or the physician can choose a dual-protease-inhibitor arm. In order to qualify for this option, they must not have taken abacavir previously for longer than eight weeks. This protocol is open to adults and adolescents (age 13 and above). Protocol 30012 is an open-label clinical trial to determine the effect of amprenavir on lipid metabolism (hyperlipidemia and lipodystrophy) in subjects experiencing these adverse effects and who are not failing current antiretroviral therapy. It is open to adults and adolescents. Also, when physicians call, they may be referred to ACTG protocols (398 and 400) with amprenavir treatment arms, in case they could send their patient to a local ACTG site. Also, physicians with pediatric patients may be referred to a Glaxo Wellcome phase III pediatric trial (for patients not previously treated with protease inhibitors). This referral will be a suggestion, not a requirement. "Regardless of which option physicians and patients opt for, patients need to have received prior treatment with at least one protease inhibitor in addition to fulfilling other standard criteria. Patients will also be strongly encouraged to start at least one other anti-HIV agent that they have not previously used." (Quoted from press statements issued jointly by the two companies). Over 700 patients have received amprenavir so far through clinical trials. Patients interested in entering this program should have their physician call the Agenerase/Amprenavir Early Access Program, 800-248-9757, 8 a.m. to 6 p.m. Eastern time, Monday through Friday. Successful Standard Treatment for Lipid Abnormalities: Experience with 44 Patients By John S. James On September 26 The Lancet published a research letter on a study at Regions Hospital in St. Paul, Minnesota on the treatment of abnormal cholesterol and triglyceride levels in persons receiving HIV protease inhibitors(1); the same team had published an early report of coronary artery disease associated with protease inhibitors(2) (see "Metabolic Changes: Concerns About Heart, Circulatory Risks," AIDS Treatment News #295, May 15, 1998). Patients who volunteered for this study were treated according to the guidelines of the National Cholesterol Education Program (NCEP), under the care of a lipid specialist. All of them continued to receive protease inhibitors. Twenty patients with milder lipid abnormalities were first treated with diet and exercise alone; 12 of the 20 were judged to be treatment failures and started on lipid-lowering drugs (gemfibrozil and/or atorvastatin). Of the patients who were treated with both of these drugs and who had the highest lipid levels, cholesterol was reduced by 30%, and triglycerides by 60%, over six months; a table in the article gives the actual values for the different treatment groups. The investigators believe it is likely that these changes indicate a significantly reduced risk of heart disease. The paper also noted that patients in their clinic who were receiving ritonavir and saquinavir were more likely to have lipid abnormalities than those on other protease inhibitors. "The NCEP guidelines advise caution in use of gemfibrozil and statins together due to a concern about increased risk for myopathy. In addition, there is concern about an increased risk for toxicity when atorvastatin is used in combination with drugs that interfere with the cytochrome p450 metabolic pathway in the liver (such as protease inhibitors). We saw no instances of myopathy, raised creatine kinase of liver enzymes, or adverse virological events among these patients." The authors conclude that "our preliminary data suggest that management of raised lipids associated with protease inhibitors can be done carefully following NCEP guidelines." References 1. Henry K, Melroe H, Huebesch J, Hermundson J, and Simpson J. Atorvastatin and gemfibrozil for protease-inhibitor- related lipid abnormalities. The Lancet. September 26, 1998; volume 352, number 3133, pages 1031-1032. 2. Henry K, Melroe H, Huebesch J, and others. Severe premature coronary artery disease with protease inhibitors. The Lancet. May 2, 1998; volume 351, page 1328. San Francisco: Body Shape Changes and Lipodystrophy-- Community Forum October 19
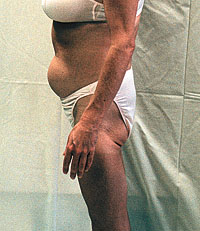 ACT UP/Golden Gate and University of California AIDS Research
Institute will sponsor a community forum on unusual body
shape changes and lipodystrophy, Monday October 19 at 7:00
p.m., at the Metropolitan Community Church, 150 Eureka
Street, San Francisco. UCSF researchers will be there to
answer questions from the public. ACT UP/Golden Gate and University of California AIDS Research
Institute will sponsor a community forum on unusual body
shape changes and lipodystrophy, Monday October 19 at 7:00
p.m., at the Metropolitan Community Church, 150 Eureka
Street, San Francisco. UCSF researchers will be there to
answer questions from the public.
Speakers are Joan Lo, M.D., Kathleen Mulligan, Ph.D., and Morris Schambelan, M.D. The program will be chaired by Mike Donnelly of ACT UP/Golden Gate, and Loren Jones of WORLD (Women Organized to Respond to Life-Threatening Diseases). The forum is free, but please RSVP (to help plan refreshments) to ari@psg.ucsf.edu, or 415-597-UCSF. For more information call ACT UP/Golden Gate, 415-252-9200. ICAAC Conference: Daily Summaries Available By John S. James The recent ICAAC conference (Interscience Conference on Antimicrobial Agents and Chemotherapy, San Diego, September 24-27, 1998) produced no big news or surprises. But there was information, among the dozens of AIDS presentations of mixed quality. Extensive daily summaries by leading AIDS treatment experts are now available at www.healthcg.com/hiv. Abacavir (Ziagen(TM)) Advisory Committee Meeting, November 2 The Antiviral Drugs Advisory Committee will hold an open public meeting for one day, Monday November 2, to discuss Glaxo Wellcome's application for approval of abacavir (Ziagen). Information about this hearing is available at the FDA Advisory Committee Information Line, 800-741-8138, or 301-443-0572 (when asked, enter code 12531 for the Antiviral Drugs Advisory Committee). A transcript of the hearing will be available later at the FDA's Web site, http://www.fda.gov; this is important because the most in-depth information about major new drugs is usually presented at these hearings. When the transcript is available (probably about two weeks after the hearing), go to the Web site and select "Dockets," then "FDA Advisory Committees Menu," then "Center for Drug Evaluation and Research (CDER)," then "Antiviral Drugs Advisory Committee." The transcripts will be listed by date, and can be downloaded in either PDF or RTF file format. (The RTF--rich text format- -is usually better for a large, text-only document, if your word processor can read it.) Prison Treatment Programs: Glaxo-Wellcome Awards 19 Grants
 On September 21 Glaxo Wellcome announced the award of 19
grants, totaling $400,000, to nonprofit organizations "to
fund innovative programs focusing on the healthcare needs of
incarcerated individuals living with HIV... The grants will
support such programs as case management services, outreach
services, educational materials and presentations, hotlines,
peer-education programs, transition programs and treatment
advocacy services." The grants ranged from $10,000 to
$40,000. One major purpose of the program is to assure
continuity of treatment access when prisoners are released. On September 21 Glaxo Wellcome announced the award of 19
grants, totaling $400,000, to nonprofit organizations "to
fund innovative programs focusing on the healthcare needs of
incarcerated individuals living with HIV... The grants will
support such programs as case management services, outreach
services, educational materials and presentations, hotlines,
peer-education programs, transition programs and treatment
advocacy services." The grants ranged from $10,000 to
$40,000. One major purpose of the program is to assure
continuity of treatment access when prisoners are released.
The 19 funded proposals were selected from among 92 submitted in response to Glaxo's RFP (see "Prison or Discharge Treatment Programs: Funding Available, Proposals Due June 29," AIDS Treatment News #296, June 5, 1996). Iscador Update: Interview with Robert Gorter, M.D. By Fred Gardner [Note: About ten years ago AIDS Treatment News noted the work of Robert Gorter, M.D., a physician from The Netherlands and associate professor at the University of California San Francisco, who was conducting AIDS research and treating patients at San Francisco General Hospital. (See "Iscador(R): Promising Experience to Date," by Denny Smith, issue #92, December 1, 1989; back issues of AIDS Treatment News are available at http://www.immunet.org/atn; search for "Iscador".) An article on Iscador also appeared in issue #2 of BETA, the Bulletin of Experimental Treatments for AIDS, published by the San Francisco AIDS Foundation ("Antivirals for HIV Infection," by Jim Palazzolo and Ron Baker, November 1988). [Ten years later, in August 1998, Dr. Gorter was interviewed by Fred Gardner, who is managing editor of Synapse, the newspaper at the University of California San Francisco Medical Center. The interview covered two topics: an update on Iscador (a mistletoe extract administered by injection, to improve the immune response), and also a large European study of cannabis (and a separate project researching a non- psychoactive marijuana ingredient called cannabidiol, or CBD). We split the interview into two articles; the one on cannabis will appear later in AIDS Treatment News. Robert Gorter, M.D., associate clinical professor at the University of California San Francisco Medical Center (Department of Family and Community Medicine), is also the medical director of the European Institute for Oncological and Immunological Research, a nonprofit with headquarters in Berlin and offices in Milan and Amsterdam. Despite his worldwide commuting and constant exposure to pathogens, the 51-year-old doctor has not been sick since 1968. He credits his good health to an extract of mistletoe marketed in Europe under the brand name "Iscador," which he is now studying in clinical trials. Fred Gardner: What is the status of your Iscador trial? Dr. Gorter: The usual steps in introducing a new product are to first do laboratory and animal studies, and then phase I, II, and III trials in humans. We had to proceed differently because Iscador is a well-known, licensed medication, available by prescription and paid for in Europe by most national healthcare systems. So it would be hard to find patients who want to participate in a randomized, placebo- controlled trial; nobody wants that when they can easily get the medication from their physician. Therefore we are doing the phase III trials in countries where mistletoe is unknown- -South Africa, Poland, and others. Gardner: Could you describe some of the research? Dr. Gorter: We are doing a randomized study in Europe of Iscador on women with cervix dysplasia--a pre-cancerous condition that is highly correlated with human papilloma virus (HPV) infection. People who develop cervix carcinoma are women who cannot clear HPV infection. With Iscador, it appears they can. We have a very high regression of cervix dysplasia in this study, which involves 200 people and 12 universities. Iscador and HIV Gardner: What have you learned about Iscador and HIV? Dr. Gorter: We have found that when patients start Iscador, they stabilize; there is no further progression, not with various markers and not clinically for many, many years. We are awaiting the results of three studies we are now conducting in South Africa. Iscador is being compared against and in addition to combination antiretroviral therapy. My impression is that in combination with standard therapy, there will be a beneficial effect because of the immune modulating activities. At the World AIDS conference in Geneva it was suggested that anti-retroviral therapy should be combined with immune modulating therapy--to not just hit the virus with toxic substances, but add supportive measures for the immune system. For More Information For more information, see the abstracts on AIDSLINE and MEDLINE; search for 'Iscador'. You can search these databases free through the World Wide Web, at http://igm.nlm.nih.gov. Also, you can contact Dr. Robert Gorter at the European Institute for Oncological and Immunological Research, email robertgorter@compuserve.com, fax +49-30-315-74444. United States Conference on AIDS, Dallas, Oct. 29 - Nov. 1 The United States Conference on AIDS, sponsored by the National Minority AIDS Council in partnership with about 20 other organizations, is expecting about 2,500 people at this year's meeting in Dallas--the largest AIDS conference in the U.S. The meeting will be held at Adam's Mark Hotel in Dallas. The conference will include over 150 workshops in 10 tracks: Care/Primary Care; Executive Director Leadership; Fundraising & Finance; Housing; International Issues; Nutrition; Prevention; Public Policy; Special Issues; and Treatment & Research. Also there will be 16 day-long Institutes on October 29. For registration information, contact Harry Williams, 202- 483-6622 ext. 343 or email lwilliam@nmac.org. For media pre- registration, contact Peter Velasco, 202-483-6622 ext. 327, or email pvelasco@nmac.org. San Francisco: POZ Life Expo, October 16-17
 POZ Life Expo 1998, a free "quality of life consumer expo for
everyone impacted by AIDS," will be held October 16-17 at the
Bill Graham Auditorium in San Francisco, 10 a.m. to 5 p.m. on
Friday, and 10 a.m. to 4 p.m. on Saturday. Although heavily
commercial, the event includes health screenings and useful
information. Contact co-host AIDS, Medicine & Miracles, 800-
875-8770, ext. 1. POZ Life Expo 1998, a free "quality of life consumer expo for
everyone impacted by AIDS," will be held October 16-17 at the
Bill Graham Auditorium in San Francisco, 10 a.m. to 5 p.m. on
Friday, and 10 a.m. to 4 p.m. on Saturday. Although heavily
commercial, the event includes health screenings and useful
information. Contact co-host AIDS, Medicine & Miracles, 800-
875-8770, ext. 1.
AIDS Treatment News Published twice monthly Subscription and Editorial Office: P.O. Box 411256 San Francisco, CA 94141 800/TREAT-1-2 toll-free U.S. and Canada 415/255-0588 regular office number Fax: 415/255-4659 E-mail: aidsnews@aidsnews.org
Editor and Publisher: John S. James Associate Editor: Tadd T. Tobias Reader Services: Tom Fontaine and Denny Smith Operations Manager: Danalan Richard Copeland Statement of Purpose: AIDS Treatment News reports on experimental and standard treatments, especially those available now. We interview physicians, scientists, other health professionals, and persons with AIDS or HIV; we also collect information from meetings and conferences, medical journals, and computer databases. Long-term survivors have usually tried many different treatments, and found combinations which work for them. AIDS Treatment News does not recommend particular therapies, but seeks to increase the options available. Subscription Information: Call 800/TREAT-1-2 Businesses, Institutions, Professionals: $270/year. Includes early delivery of an extra copy by email. Nonprofit organizations: $135/year. Includes early delivery of an extra copy by email. Individuals: $120/year, or $70 for six months. Special discount for persons with financial difficulties: $54/year, or $30 for six months. If you cannot afford a subscription, please write or call. Outside North, Central, or South America, add air mail postage: $20/year, $10 for six months. Back issues available. Fax subscriptions, bulk rates, and multiple subscriptions are available; contact our office for details. Please send U.S. funds: personal check or bank draft, international postal money order, or travelers checks. ISSN # 1052-4207 Copyright 1998 by John S. James. Permission granted for noncommercial reproduction, provided that our address and phone number are included if more than short quotations are used. |
© 1997-98 BEI